
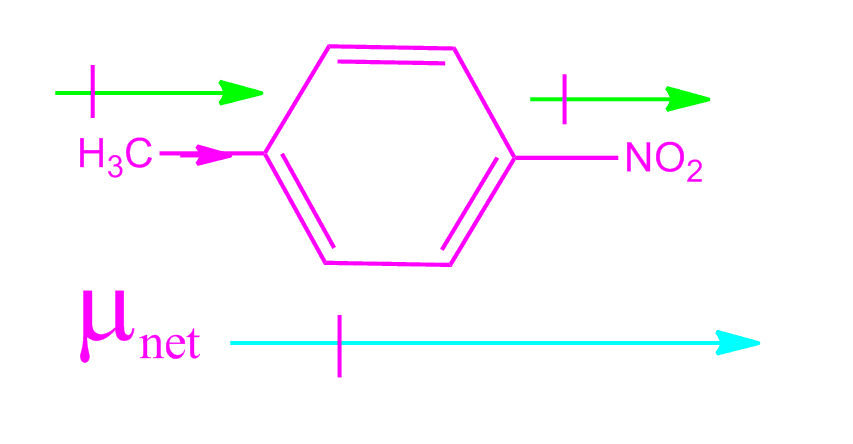
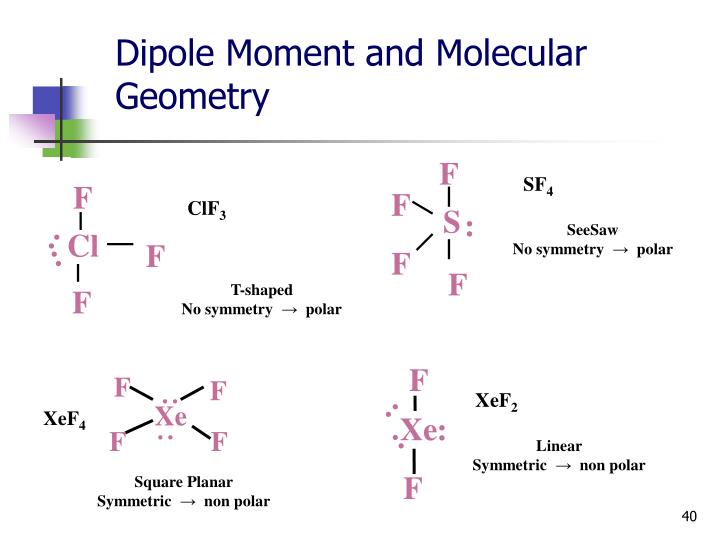
Jacox, Vibrational and Electronic Energy Levels of Polyatomic Transient Molecules, J. , Tables of Molecular Vibrational Frequencies, Consolidated Volu Maryott "Selected Values of electric dipole moments for molecules in the gas phase" NSRDS-NBS10, 1967 Chlorine trifluoride has three polarized bonds and they combine to produce a small molecular dipole along the Cl-F bond. The dipole moment acts in the direction of the vector quantity.
Please address comments about this page to Thakkar, T Wu "How well do static electronic dipole polarizabilities from gas-phase experiments compare with density functional and MP2 computations?" J. The dipole moment of a molecule can be calculated by Equation 1: i qiri where is the dipole moment vector qi is the magnitude of the ith charge, and ri is the vector representing the position of ith charge. NIST does not necessarily endorse the views expressed, or concur with the facts presented on these sites.įurther, NIST does not endorse any commercial products that may be mentioned on these sites. There may be other web sites that are more appropriate for your purpose.

No inferences should be drawn on account of other sites being referenced, or not, from this page. We have provided these links to other web sites because they may have information that would be of interest to you. You are here: Experimental > One molecule all propertiesĮxperimental data for ClF 3 (Chlorine trifluoride)Ĭhlorine fluoride Chlorine fluoride (ClF3) Chlorine trifluoride Chlorotrifluoride Trifluorure de chlore īy selecting the following links, you may be leaving NIST webspace.


 0 kommentar(er)
0 kommentar(er)
